Anode and Cathode in Electrolysis
2 H 2 O 2 e-H 2 2 OH-Anode. The electrolyte conducts electricity for electrolysis to occur.
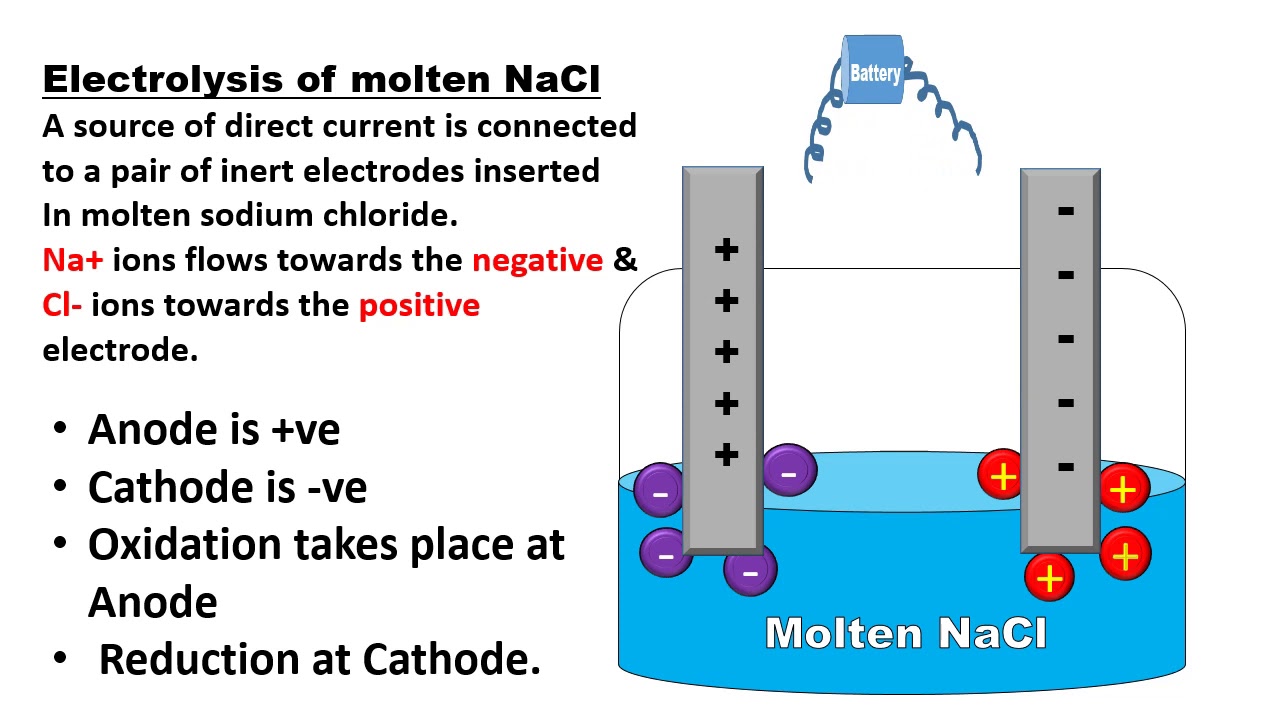
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
Many devices have other electrodes to control operation eg base gate control grid.

. The slideshow shows what happens during the purification of copper by electrolysis. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. The chemical change is one in which the substance loses or gains an electron oxidation or reduction.
Anions move through the barrierbridge toward the electrode where oxidation is occurring. Releasing electrons to the anode. The electrolysis takes place at a much lower potential than pure water 24V.
A cathode is a negatively charged electrode. Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current. The cathode is the current that leaves the electrodes or cathode is a result of reduction reaction taking place in an electrolyte mixture.
The process is carried out in an electrolytic cell an apparatus consisting of positive and negative electrodes held apart and dipped into a solution containing positively and. The reaction between the two elements in an electrolytic cell is a reduction-oxidation -- or redox -- reaction. When voltage is applied hydrogen is produced at the cathode and oxygen at the anode.
Electroplating and electrolysis welding cathodic protection. In the electrolysis process electrolyzers use electricity for water-splitting. According to the equations for the two half-reactions the indicator should turn yellow at the anode and blue at the cathode.
Water Electrolysis in the Presence of a Base pH higher than 7 Additional hydroxyl ions release their electrons to anode while electrons at the cathode oxidize water molecules near it. Electrolysis is used to drive an oxidation-reduction reaction in a direction in which it does not occur spontaneously. In a vacuum tube or a semiconductor having polarity diodes electrolytic capacitors the anode is the positive electrode and the cathode the negative.
Form at the cathode. Water electrolysis relies on the application of an electric current through an anode and cathode inserted in the electrolyte to provide the required energy to break the hydrogen and oxygen bond. The less noble metal in a reaction will be the anode.
The electrode where reduction is occurring is represented by a positive sign. The more noble metal in the reaction will always be the cathode. Electrolysis is a technique that uses a direct electric current DC.
Half reactions of electrolysis in the presence of a base are-. Cathode Anode and Electrolyte. According to the general definition an electrode is a substance that helps in electricity conduction wherein the electric current either leaves or enters the non-metallic medium such as an electrolytic cell.
So what does that mean. Electrolysis process by which electric current is passed through a substance to effect a chemical change. At the cathode water is split to form H2 and releases hydroxide anions which pass through the diaphragm and recombine at the anode to form O2.
The main components of an electrochemical cell are the anode the cathode and the electrolyte. Bubbles of oxygen gas O form at the anode and bubbles of hydrogen gas H 2 2. 2 H 2 O O 2 4 H 4 e-Faradays Law.
The H ions called cations move toward the cathode negative electrode and the OH- ions called anions move toward the anode positive electrode. To flow when a voltage is applied. JES is the flagship journal of The Electrochemical Society.
During electrolysis the anode loses mass as copper dissolves and the cathode gains mass as copper is deposited. The cathode is made of pure copper or a support metal such as stainless steel. Here electrons are released from the electrode and the surrounding solution is reduced.
The anode and cathode of a voltaic cell are separated into two half-cells and connected by a metal wire. Then they head towards the cathode. Electrolysis involves the manipulation of chemical reactions based on their electric potential.
Different electrolyzers function in different ways mainly due to the different type of electrolyte. Published continuously from 1902 to the present JES remains one of the most highly-cited journals in electrochemistry and solid-state science and technology. Electrolysis involves passing an electric current through either a molten salt or an ionic solution.
Low-temperature CO2 electrolysis represents a potential enabling process in the production of renewable chemicals and fuels notably carbon monoxide formic acid ethylene and ethanol. An electrochemical reaction occurs which is 7080 efficient and is the most established well-known commercially available technology for water-splitting. The ions are forced to undergo either oxidation at the anode or reduction at the cathode.
The electrons enter the device through the cathode and exit the device through the anode. Reduction occurs at the cathode. The anode and cathode are separated by a diaphragm separating hydrogen and oxygen gases and preventing them from mixing up again.
Like fuel cells electrolyzers consist of an anode and a cathode separated by an electrolyte. The bubbles are easily seen. The electrolysis can be done using two weighed copper strips.
Electrons flow in the external circuit from the. The process of electrolysis sees electrons being stripped from the anode. And in order to separate the different gases formed at the cathode and anode respectively the use of a.
The anode is the site where. While the basis remains the same different electrolysis methods including polymer electrolyte membrane electrolyzer alkaline electrolyzer and solid. The anode is a positively charged electrode.
The anode consists of an unrefined sample of the metal. Before we learn about the terms cathode and anode it is important to understand what an electrode is. The reaction takes place in a unit called an electrolyzer.
Commercially electrolytic cells are used in electrorefining and electrowinning of several non-ferrous metals. Likewise the cathode reduces sodium ions Na which accept electrons from the cathode and deposits on the cathode as sodium. In practice electrolysers consist of several interconnected electrolysis cells also called stacks.
In electrochemical cells the cathode is the site where reduction occurs. The two common terms we hear is cathode and anode. This is to confirm that the mass gained at the cathode is.
The splitting occurs in two partial reactions that take place at the two electrodes cathode - and anode in the electrolysis cell.

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative

Chemistry Subject Nickel Electroplating System Electronic Circuit Projects Chemistry Electroplating
0 Response to "Anode and Cathode in Electrolysis"
Post a Comment